In August of last year, we sent our cosmetic and OTC drug clients a link to a Hyman, Phelps and McNamara Law Blog Post about a lawsuit against FDA that sought to force the agency to finalize the tentative final monograph on hand sanitizers that has been “tentative” since 1994. This week FDA issued four warning letters to companies promoting hand sanitizers and other products as effective again MRSA (methicillin-resistant Staphylococcus aureus bacteria). FDA issued a press announcement about these letters due to the concern that the public could be misled and subsequently harmed by the MRSA claims. I find the warning letters interesting because of what we can learn about the regulation of over the counter drug products, particularly those under review by FDA or subject to tentative final monographs.
One of these warning letters went to Oh So Clean, Inc, doing business as CleanWell Company, which markets thymol-based hand sanitizers and sanitizing wipes. (Thymol is a constituent in the herb thyme.) The warning letter demonstrates how FDA can enforce the stipulations of over the counter drug monographs that remain tentative. The warning letter results from FDA inspection of the company in August of 2010. At that time, CleanWell Company received a 483 letter detailing the violations FDA observed during the inspection. The warning letter issued last week notes that the company’s September response to the 483 letter “lacks sufficient corrective actions” and details FDA’s findings that the products are unapproved new drugs.
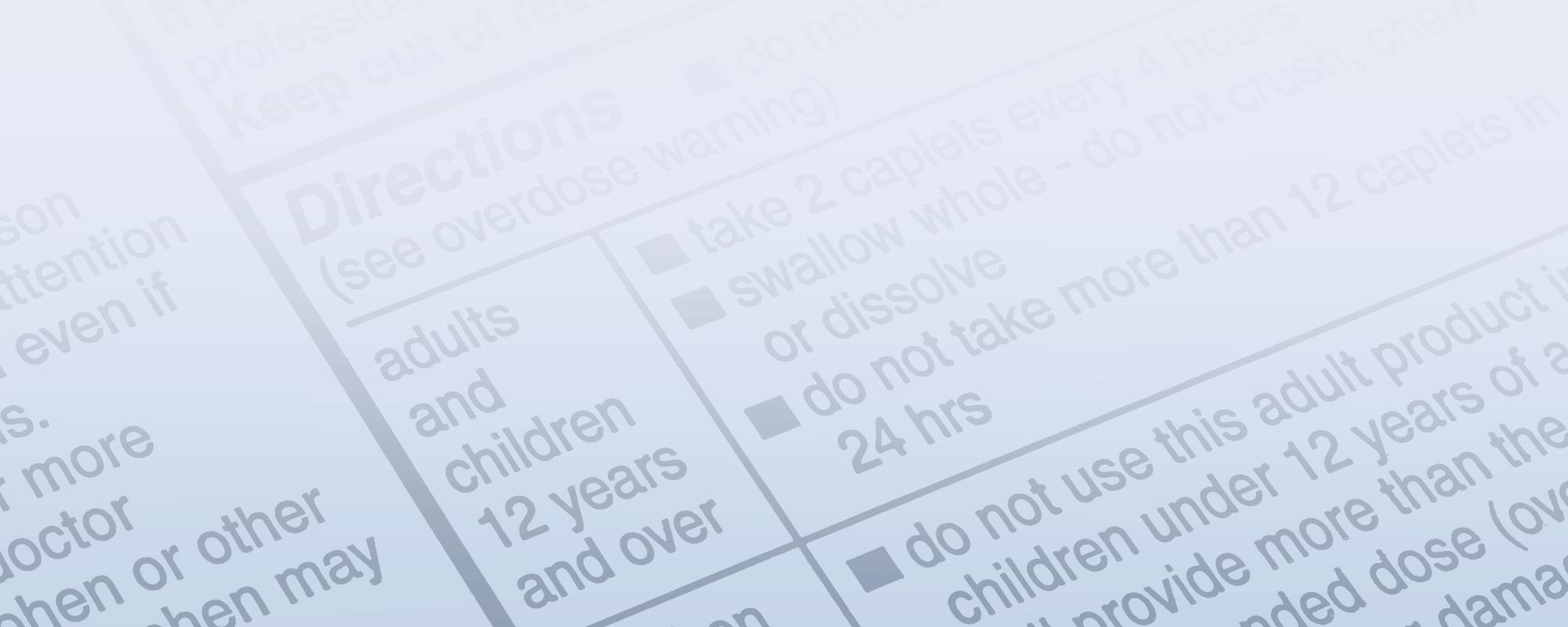
Based on the product labels and the company’s website, FDA categorizes the CleanWell products as “OTC topical antimicrobials” and cites the tentative final monographs (TFMs) for OTC healthcare antiseptics and OTC first aid antiseptics as the governing regulations for these products. Interestingly, the response to an inquiry our company made to FDA in 2009 listed only the healthcare TFM as pertaining to hand sanitizers. Nevertheless, both of these monographs are being evaluated under FDA’s OTC Drug Review process and the April 18 warning letter notes that “Pending a final monograph, the agency does not object to the marketing of OTC drugs that meet the formulation and labeling requirements described in the TFM.” Unfortunately, the CleanWell “products’ formulation and labeling are not consistent with any of these TFMs.” You can review the TFMs at http://tinyurl.com/3pxx464.
So what were the problems with these products?
First, the drug facts boxes identified Thymus vulgaris oil (thymol 0.05%) as the only active ingredient. The pertinent TFMs do not list this as an approved active ingredient but the First Aid Antiseptic TFM lists thymol in combination with eucalyptus, menthol, methyl salicylate and alcohol as an acceptable active ingredient. In addition, the product claims go beyond what is permitted by the tentative final monographs for antiseptic products. In particular, the claims of effectiveness against MRSA are non-monograph. The only way to make claims outside of those detailed in the monographs is to undertake clinical trials in order to gain FDA approval for new over the counter drug claims.
Even if thymol alone were an acceptable active ingredient for this type of OTC product, the company’s own website and other labeling state that active ingredient used in the products is a blend of essential plant oils. FDA refers to incorrect labeling such as this as “misbranding”.
The warning letter further cites violations of good manufacturing practices, which render the products “adulterated” and orders the company to “cease manufacturing and distributing all your unapproved new drug products” and to take corrective action and respond to the warning letter within 15 working days. This is undoubtedly a real blow to products that were available in several well-known retail outlets.
Similar problems were noted in the other warning letters about MRSA claims—simple failure to follow the monographs, which detail how to formulate and label over the counter drug products. It is our view that timely and competent review of the product labels and claims could have helped these companies identify the issues cited by FDA and remain active in the marketplace. Is your product compliant with applicable FDA regulations or has a slight tweak of the marketing materials skewed your claims beyond those permitted? Let us help you ensure compliance so your marketing plans are not disrupted by FDA enforcement activities.