Update May 22, 2016: Here is a brief summary of the changes to the Nutrition Facts Box:
The side by side graphic below shows the current and soon to be required versions of the Nutrition Facts Box. The changes also apply to Supplement Facts Boxes. While the changes include much larger font sizes for serving size and calories listings and a new required listing for added sugars, the large calorie chart footnote has been eliminated so that the average overall size of the facts box has not changed.
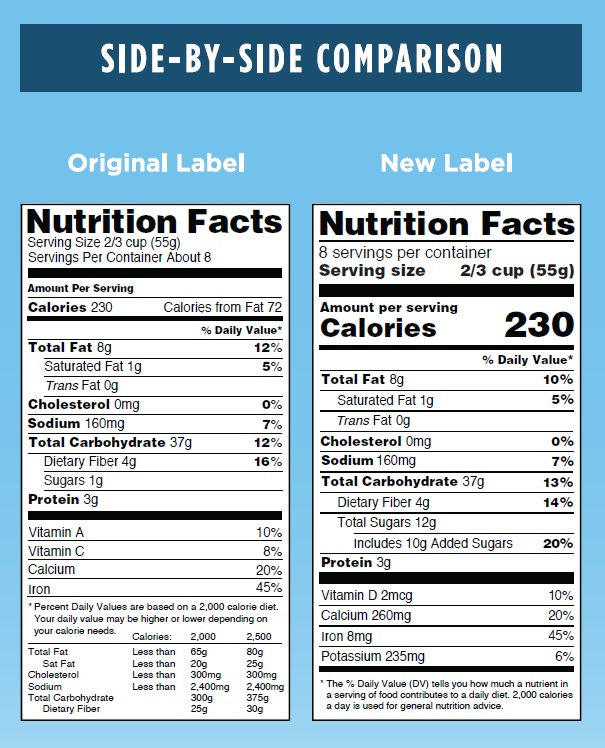
This image is taken from the FDA webpage.
In addition to the numerous changes to the look of the graphics, the percent daily values (DVs) have changed for many nutrients. The newly required nutrient, “Added sugars” also has a daily value, 50 grams and at last the B vitamin Choline has been assigned a daily value of 550 mg. Here are a few more highlights: The DVs for total carbohydrates, sodium and many of the B vitamins have been reduced while those for total fat, dietary fiber, vitamin C, vitamin D, calcium and magnesium have been increased.
FDA also announced changes to the serving sizes required for use in food product labeling. New items such as tortillas and wonton wrappers have been added. The serving size for most beverages has increased from 8 to 12 ounces.
Another important change to note is that all product packages that contain at least 200% and up to 300% of the applicable serving size must list nutrition information for the serving size and the entire container as shown in this image from FDA’s announcement:
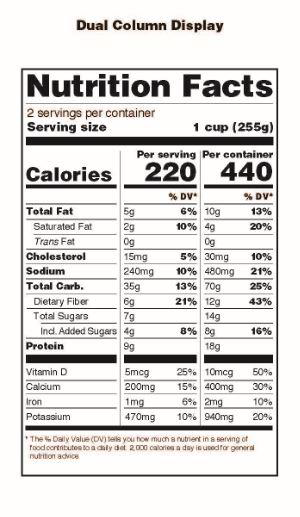
These changes also apply to the Supplement Facts Boxes on dietary supplement products but the most pronounced changes such as the large size for Calories and the listing of ‘Added Sugars’ will not affect the majority of dietary supplement products.
It is interesting to note that the preamble to the final rule discusses many comments that were received about protein declaration and protein quality and digestibility. In the discussion of and response to these comments, FDA reiterates the requirement to correct protein values for products whose quality/digestibility scores are less than 40%. This affects some vegetarian protein products that do not rely on soy as their primary source of protein.
The new rules become effective on July 26, 2016 but compliance is not required until July 26, 2018 for manufacturers with $10 million or more in annual food sales and July 26, 2019 for manufacturers with less than $10 million in annual food sales. If you are updating labels or planning a new product introduction after July 25, 2016, you may wish to consider working with the new format.
Update May 20, 2016: Today FDA announced the final rule for changes to the Nutrition Facts Box. The new rules become effective on July 26, 2016 but compliance is not required until July 26, 2018 for manufacturers with $10 million or more in annual food sales and July 26, 2019 for manufacturers with less than $10 million in annual food sales.
Most of the proposed changes that we discussed in our February 2014 post below are part of the final rule. The look of the revised facts box is a little different than proposed in 2014 (see image below). We will update this post as we review the new rules.
Original Post February 27, 2014: You’ve no doubt heard the news today that FDA is proposing a number of changes to the Nutrition Facts and Dietary Supplement Facts boxes that appear on US products. The proposed rules are based on review of the state of Americans’ health, dietary patterns and newer understandings of nutritional science. The official announcement will be made at 3:00 pm Eastern Time and the rule is only proposed at this time and will not be in effect for about two years but I’ve spent some time this morning reviewing the proposed rule and thought you might be interested in a few of the highlights:
- The number of calories present in food and dietary supplement products will be much larger—larger than the current “Nutrition Facts” title. The number of servings per container will be much larger as well. (see image below).
- The “%Daily Value” declaration would be moved to the left side of the panel.
- There is a proposal to eliminate the declaration of Calories from fats from the facts boxes
- FDA wishes to require the declaration of “added sugars” in addition to total carbohydrates and the levels of naturally-occurring sugars. This particular proposal will require excellent record keeping by manufacturers and distributors since there are no analytical methods that can differentiate between naturally occurring and added sugars.
- There are some changes proposed to the RDIs and Percent Daily Values for some nutrients and to the units used to express others. Sodium’s RDI would decrease from 2400 mg to 2300 mg per day. The fat soluble vitamins, A, D, E and K would now be expressed in milligram or microgram amounts rather than the current (and confusing)
International Units. The RDI for calcium would be increased from 1000 mg to 1300 mg. See table below for details except that for some reason sodium was left out of this table. - The proposed rule would require the mandatory declaration of vitamin D and potassium, retain the current mandatory declaration of calcium and iron and make the declaration of vitamin A and vitamin C voluntary.
- FDA also proposes that records be kept in support of label declarations of dietary fiber, sugars that undergo fermentation (including those in yeasted breads), various forms of vitamin E, and folate and folic acid. The proposal would require maintenance of such records for two years.
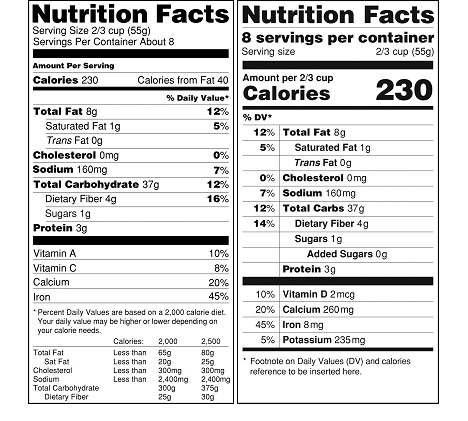
It is important for manufacturers to consider how the proposed rule would affect their businesses and to consider submitting comments to FDA regarding the proposed rule. We will provide updates and insights as the process moves forward and in the meantime can help you with the current regulations




